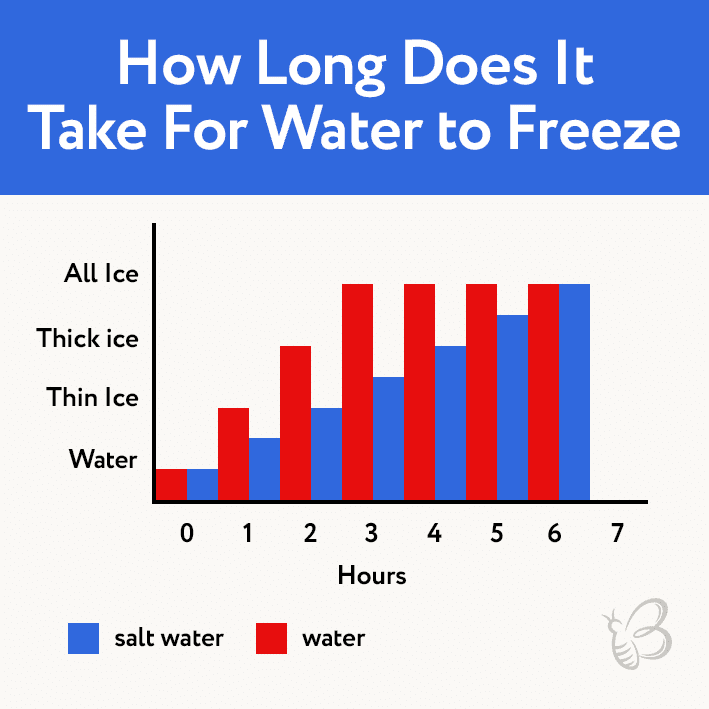
The time it takes for water to freeze depends on various factors such as the volume of water, its initial temperature, the presence of impurities, and the surrounding environment. Generally, the larger the volume of water, the longer it takes to freeze. Warmer water takes longer to freeze than cold water. Impurities like salt or sugar can also affect the freezing time, as they can lower the freezing point of water. Additionally, the surrounding environment, such as the presence of wind or the temperature of the surrounding air, can influence the freezing process.
Understanding the freezing time of water is important for various reasons. It is crucial for predicting and preventing freezing-related hazards, such as icy roads and frozen pipes. It also plays a role in food preservation and industrial processes involving freezing and thawing.
To further explore the topic of water freezing time, we can delve into specific scenarios and scientific principles governing the freezing process. We can also discuss practical applications and implications of this knowledge in different fields.
- Capturing Lifes Moments Stunning Photos From Canon 80d
- Exploring The Exquisite Flavors Of Tr Oriental Menu
How Long Does It Take for Water to Freeze At?
Understanding the factors that influence the freezing time of water is crucial for various reasons. Here are eight key aspects to consider:
- Volume
- Initial temperature
- Impurities
- Surface area
- Agitation
- Pressure
- Nucleation sites
- Surrounding environment
These aspects are interconnected and influence the freezing process in different ways. For instance, increasing the volume of water generally prolongs the freezing time, while a higher initial temperature requires more energy to remove before freezing can occur. Impurities can lower the freezing point, affecting the time it takes for water to freeze completely. The surface area, agitation, and pressure can influence the rate of heat transfer, which in turn affects the freezing time. Nucleation sites, such as dust particles or scratches on the container's surface, can provide starting points for ice crystal formation, potentially accelerating the freezing process. Finally, the surrounding environment, including the ambient temperature and the presence of wind, can significantly impact the freezing time.
1. Volume
Volume plays a significant role in determining how long it takes for water to freeze. The larger the volume of water, the longer it takes to freeze because there is more thermal energy that needs to be removed before the entire body of water reaches its freezing point and solidifies.
- Discover The Comfort Of An Outdoor Glider Chair With Ottoman
- Greg Lemond Vs Lance Armstrong Cycling Legends And Their Controversial Legacies
- Surface Area
The surface area of the water also affects the freezing time. A larger surface area allows for more heat to be transferred to the surrounding environment, which can accelerate the freezing process. For example, a shallow pool of water will freeze faster than a deep pool of the same volume.
- Container Material
The material of the container holding the water can also influence the freezing time. Materials with high thermal conductivity, such as metals, allow heat to transfer more easily, leading to faster freezing. In contrast, materials with low thermal conductivity, such as plastics or wood, can slow down the freezing process.
- Agitation
Agitating the water can also affect the freezing time. Stirring or circulating the water helps to distribute heat more evenly throughout the liquid, which can slow down the freezing process. For example, a glass of still water will freeze faster than a glass of water that is being stirred.
- Impurities
The presence of impurities in the water can also affect the freezing time. Impurities, such as salt or sugar, can lower the freezing point of water, which means that the water will take longer to freeze. For example, salt water freezes at a lower temperature than pure water.
Understanding the relationship between volume and freezing time is important for various applications, such as designing efficient refrigeration systems, predicting the freezing time of water in natural water bodies, and optimizing industrial processes that involve freezing and thawing.
2. Initial temperature
The initial temperature of water plays a crucial role in determining how long it takes to freeze. The higher the initial temperature, the longer it takes for the water to reach its freezing point and solidify. This is because more thermal energy needs to be removed from the water to lower its temperature to the freezing point.
For example, if you have two identical glasses of water, one at room temperature (20C) and the other at 5C, the glass of water at 5C will freeze faster than the glass of water at room temperature. This is because the water at 5C is closer to its freezing point and requires less energy to be removed before it can freeze.
Understanding the relationship between initial temperature and freezing time is important for various applications, such as:
- Predicting the freezing time of water in natural water bodies, such as lakes and rivers
- Designing efficient refrigeration systems
- Optimizing industrial processes that involve freezing and thawing
3. Impurities
The presence of impurities in water can significantly affect how long it takes to freeze. Impurities are substances that are dissolved or suspended in water, such as salt, sugar, or minerals. These impurities can lower the freezing point of water, which means that the water will take longer to freeze.
- Salt
Salt is one of the most common impurities found in water. It can lower the freezing point of water by up to 2 degrees Celsius (3.6 degrees Fahrenheit). This means that a glass of salt water will take longer to freeze than a glass of pure water.
- Sugar
Sugar is another common impurity found in water. It can lower the freezing point of water by up to 1 degree Celsius (1.8 degrees Fahrenheit). This means that a glass of sugary water will take longer to freeze than a glass of pure water.
- Minerals
Minerals are also common impurities found in water. They can lower the freezing point of water by varying amounts, depending on the type of mineral. For example, calcium carbonate can lower the freezing point of water by up to 0.5 degrees Celsius (0.9 degrees Fahrenheit).
The presence of impurities in water can have a significant impact on how long it takes to freeze. This is important to consider in a variety of applications, such as food preservation, industrial processes, and weather forecasting.
4. Surface area
The surface area of water exposed to the surrounding environment plays a crucial role in determining how long it takes to freeze. This is because heat transfer occurs more rapidly across a larger surface area. In the context of water freezing, a greater surface area allows for more efficient heat dissipation, leading to faster freezing.
- Exposed Surface Area
The amount of water surface exposed to the surrounding air directly influences the freezing rate. A larger exposed surface area allows for greater heat exchange, promoting faster freezing. For instance, a shallow pond with a large surface area will freeze more quickly than a deep pond with a smaller surface area.
- Container Shape
The shape of the container holding the water can also impact the surface area exposed to the environment. Containers with a larger surface area relative to their volume, such as wide, shallow pans, facilitate faster freezing compared to narrow, deep containers.
- Agitation and Circulation
Introducing agitation or circulation to the water can increase the effective surface area for heat transfer. Stirring or circulating the water exposes more of its volume to the surrounding environment, accelerating the freezing process.
- Environmental Factors
The temperature and humidity of the surrounding environment can influence the surface heat transfer rate. In colder environments with lower humidity, the temperature gradient between the water and the air is greater, promoting faster heat dissipation and freezing.
Understanding the relationship between surface area and freezing time is essential in various applications, including refrigeration, industrial freezing processes, and predicting ice formation in natural water bodies.
5. Agitation
Agitation plays a significant role in determining how long it takes for water to freeze. Agitation refers to the disturbance or movement of water, which affects the rate of heat transfer and influences the freezing process.
When water is agitated, it exposes more of its surface area to the surrounding environment. This increased surface area allows for greater heat dissipation, as heat can escape more readily from the water's surface. As a result, agitated water freezes more quickly compared to still water.
A practical example of this phenomenon can be observed when comparing the freezing time of a glass of still water to that of a glass of water that is being stirred. The stirred water will freeze faster due to the increased agitation and surface area exposure.
Understanding the connection between agitation and freezing time is important in various applications. For instance, in industrial freezing processes, agitation is employed to accelerate the freezing rate, ensuring efficient and uniform freezing of products. Similarly, in refrigeration systems, agitation helps maintain a consistent temperature and prevents the formation of ice crystals, contributing to optimal cooling performance.
6. Pressure
Pressure exerts a profound influence on how long it takes for water to freeze at. Understanding this relationship is crucial in various scientific and practical applications. Here are four key facets to consider:
- Effect on Freezing Point
Pressure can alter the freezing point of water. As pressure increases, the freezing point of water decreases. This means that water under high pressure will freeze at a lower temperature compared to water at atmospheric pressure. This phenomenon is observed in deep-sea environments, where water remains liquid despite the freezing temperatures due to the immense pressure exerted by the overlying water.
- Impact on Ice Formation
Pressure can affect the formation and structure of ice crystals. Under high pressure, water molecules are forced closer together, which can hinder the formation of ice crystals and result in the formation of amorphous ice. Amorphous ice has a disordered structure compared to crystalline ice and exhibits different physical properties.
- Role in Glacier Dynamics
In glaciers, the immense pressure exerted by the overlying ice can influence the freezing and melting processes. The pressure gradient within a glacier can lead to variations in the freezing point, affecting the ice flow and dynamics. Understanding the pressure-freezing relationship is crucial for modeling glacier behavior and predicting their response to climate change.
- Applications in Ice Engineering
The knowledge of the pressure-freezing relationship finds applications in ice engineering, particularly in the design of ice rinks and ice roads. By controlling the pressure applied to the ice surface, engineers can manipulate the freezing process to achieve the desired ice properties and performance.
In summary, pressure is an important factor that influences how long it takes for water to freeze at. Its effects on freezing point, ice formation, glacier dynamics, and ice engineering applications highlight the significance of understanding this relationship in various scientific and practical contexts.
7. Nucleation sites
In the context of "how long does it take for water to freeze at;", nucleation sites play a crucial role in determining the freezing time. Nucleation sites are microscopic imperfections or particles suspended in water that provide a surface for water molecules to attach to and form ice crystals. The presence and characteristics of these nucleation sites significantly influence the freezing process.
- Type and Concentration
The type and concentration of nucleation sites in water can impact the freezing time. Certain impurities, such as dust particles or organic matter, can act as nucleation sites. A higher concentration of nucleation sites provides more surfaces for ice crystal formation, leading to faster freezing.
- Size and Shape
The size and shape of nucleation sites can also affect the freezing process. Larger nucleation sites can promote faster freezing, as they provide a more stable surface for ice crystal growth. The shape of the nucleation site can influence the orientation and growth pattern of ice crystals.
- Surface Properties
The surface properties of nucleation sites, such as their wettability and charge, can influence the freezing process. Hydrophilic surfaces, which attract water molecules, can promote faster freezing compared to hydrophobic surfaces. The charge of the nucleation site can also affect the interaction with water molecules and influence the ice crystal growth.
- Environmental Factors
Environmental factors, such as temperature and pressure, can influence the effectiveness of nucleation sites. At lower temperatures, nucleation sites become more effective in promoting freezing. Pressure can also affect the behavior of nucleation sites and the freezing process.
Understanding the role of nucleation sites is crucial for various applications, including cryopreservation, food freezing, and cloud seeding. By manipulating nucleation sites, it is possible to control the freezing process and achieve desired outcomes, such as preventing ice damage or promoting faster freezing for efficient food preservation.
8. Surrounding environment
The surrounding environment plays a crucial role in determining how long it takes for water to freeze at. Several environmental factors can significantly influence the freezing process, including:
- Temperature: The ambient temperature is a primary factor affecting the freezing time of water. The colder the surrounding environment, the faster water will freeze. This is because lower temperatures provide less thermal energy for water molecules to overcome in order to transition into a solid state.
- Humidity: The humidity level of the surrounding air can also affect the freezing time of water. In drier environments, water tends to evaporate more quickly, which can lead to a decrease in the overall volume of water and a faster freezing process. Conversely, in humid environments, the presence of more water vapor in the air can slow down evaporation and prolong the freezing time.
- Wind: The presence of wind can accelerate the freezing process. Wind increases the rate of heat transfer from the water's surface to the surrounding air, which can lead to faster cooling and freezing. This effect is particularly pronounced in cold, windy environments.
- Insulation: The presence of insulation around the water can slow down the freezing process. Insulation materials, such as foam or blankets, create a barrier that reduces heat transfer between the water and the surrounding environment. This can be useful in situations where it is desirable to prevent water from freezing, such as in pipes or outdoor water features during cold weather.
Understanding the connection between the surrounding environment and the freezing time of water is important for a variety of practical applications. For example, in cold climates, it is crucial to take precautions to prevent water pipes from freezing, which can lead to burst pipes and water damage. Additionally, in food processing and preservation, controlling the surrounding environment can help to optimize the freezing process and maintain the quality of frozen products.
Overall, the surrounding environment is a key factor that can significantly influence how long it takes for water to freeze at. By understanding the relationship between these environmental factors and the freezing process, it is possible to develop strategies to control and optimize freezing for various applications.
FAQs on "How Long Does It Take for Water to Freeze At?"
This section addresses commonly asked questions and misconceptions surrounding the topic of water freezing time.
Question 1: Why does water take longer to freeze in a freezer than in nature?
Answer: While it may seem counterintuitive, water can actually freeze faster in nature than in a freezer. This is because in nature, water is exposed to a wider range of freezing nuclei, such as dust particles and impurities, which provide surfaces for ice crystals to form. In a freezer, the environment is more controlled and there are fewer freezing nuclei present, which can slow down the freezing process.
Question 2: Can water freeze instantaneously?
Answer: Under certain conditions, water can undergo a process called "homogeneous nucleation," where ice crystals form without the presence of any impurities or freezing nuclei. This process is very rare and requires extremely cold temperatures and high pressure. However, it is possible to achieve near-instantaneous freezing in laboratory settings using specialized techniques.
Question 3: What factors affect the freezing time of water?
Answer: The freezing time of water is influenced by several factors, including the volume of water, the presence of impurities, the surrounding temperature, and the surface area exposed to the environment. Larger volumes of water take longer to freeze, impurities can lower the freezing point, and higher surrounding temperatures slow down the freezing process. Increasing the surface area can accelerate freezing.
Question 4: Why does boiling water freeze faster than cold water?
Answer: The misconception that boiling water freezes faster than cold water is known as the "Mpemba effect." While there is some evidence to support this phenomenon under specific conditions, it is generally not true. In most cases, cold water will freeze faster than boiling water.
Question 5: Can freezing water damage pipes?
Answer: Yes, freezing water can cause pipes to burst. When water freezes, it expands in volume, which can put pressure on the pipe walls and cause them to crack. This is a common problem in cold climates during winter.
Question 6: What is the significance of understanding water freezing time?
Answer: Understanding the freezing time of water is important for various reasons. It helps us predict and prevent freezing-related hazards, such as icy roads and frozen pipes. It also has applications in food preservation, industrial processes, and scientific research.
In summary, the freezing time of water is influenced by a combination of factors and can vary depending on the specific conditions. By understanding these factors, we can better control and predict the freezing process for various practical applications.
Transition to the next article section:
Now that we have explored the topic of water freezing time in detail, let's move on to discussing the importance of water in various scientific and industrial contexts.
Tips on "How Long Does It Take for Water to Freeze At?"
Understanding the factors that influence the freezing time of water is crucial for various applications. Here are five practical tips to consider:
Tip 1: Consider the Volume of Water
The larger the volume of water, the longer it will take to freeze. Plan accordingly when dealing with large bodies of water, such as pools or ponds, especially in cold climates.
Tip 2: Pay Attention to Initial Water Temperature
Water with a higher initial temperature will take longer to freeze. Pre-cooling water can significantly reduce freezing time, particularly in industrial or food processing settings.
Tip 3: Be Aware of Impurities and Dissolved Substances
Impurities and dissolved substances can lower the freezing point of water. Consider these factors when dealing with saltwater or water containing additives, as they may affect freezing time.
Tip 4: Manage Surface Area and Agitation
Increasing the surface area of water exposed to the environment can accelerate freezing. Agitating the water can also promote faster freezing by exposing more water to the surrounding air.
Tip 5: Control the Surrounding Environment
Colder temperatures, lower humidity, and the presence of wind can all contribute to faster freezing. Controlling the surrounding environment can be essential in applications such as refrigeration and cryopreservation.
By applying these tips, you can optimize freezing time and improve the efficiency of processes that involve water freezing in various scientific and industrial contexts.
Conclusion
Understanding the factors that influence how long it takes for water to freeze at is a valuable skill with practical applications in diverse fields. By considering volume, initial temperature, impurities, surface area, agitation, and the surrounding environment, you can control and optimize the freezing process for desired outcomes.
Conclusion
In summary, understanding the complexities of water freezing is essential in a multitude of scientific and practical applications. By delving into the various factors that influence how long it takes for water to freeze at, we gain valuable insights into controlling and optimizing freezing processes.
From the impact of volume and initial temperature to the role of impurities, surface area, agitation, and the surrounding environment, each element plays a crucial part in determining freezing time. Recognizing these factors empowers us to approach freezing-related challenges with greater precision and efficiency.
As we continue to explore the fascinating world of water freezing, new discoveries and advancements will undoubtedly emerge. This ongoing pursuit of knowledge will contribute to further advancements in diverse fields, from engineering and food preservation to climate science and beyond.
- Exploring The Grande Iced Caramel Macchiato Price A Sweet Treat Worth Sipping
- Capturing Worth The Essence Of The Photography Of Value